What Freezes After It Is Heated Up: The Science Behind Phase Changes
What if I told you that there’s an intriguing phenomenon in the world of science where something freezes after it’s been heated up?
Yes, you heard that right!
It may sound counterintuitive, but this mind-boggling concept challenges our understanding of the natural order.
Buckle up and get ready to explore the mysterious world of phase changes, as we dive into the question of what freezes after it’s heated up.
Prepare to have your curiosity sparked!
what freezes after it is heated up
Water freezes after it is heated up.
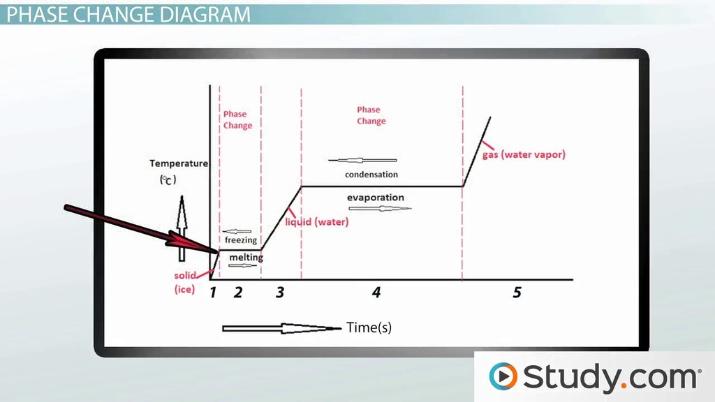
Heating water causes its temperature to rise, leading to the phase change from liquid to gas, known as boiling.
However, if the heated water is rapidly cooled down, such as placing it in a freezer or exposing it to extremely cold temperatures, it will change from a gas back into a liquid and then solidify into ice.
Therefore, water is a substance that can freeze after being heated up.
Key Points:
- Heating water causes its temperature to rise and undergo a phase change from liquid to gas.
- Rapidly cooling down heated water can cause it to change from gas back into a liquid and then solidify into ice.
- This shows that water is a substance that can freeze after being heated up.
- The phase change from liquid to gas is known as boiling.
- Placing heated water in a freezer or exposing it to extremely cold temperatures can facilitate the freezing process.
- The freezing of heated water demonstrates the unique behavior and properties of water as a substance.
what freezes after it is heated up – Watch Video
💡
Pro Tips:
1. Ice cream was invented in ancient China, where it was made by mixing heated, flavored milk with snow or ice. Despite the unusual process, it freezes into the creamy texture we know and love today.
2. Baking soda, a common ingredient found in many households, can freeze after being heated. When heated to high temperatures and rapidly cooled, baking soda transforms into a solid white powder known as sodium pyrosulfate.
3. Lava, which is molten rock expelled by a volcano, can actually freeze after being heated up. When lava cools rapidly, either due to contact with water or exposure to low temperatures, it quickly solidifies and forms various volcanic rocks.
4. Silkworm cocoons, which are typically heated during the process of silk production, have an interesting freezing property. When exposed to extremely low temperatures, such as in a freezer, the pupa inside the cocoon could enter a state of suspended animation and effectively freeze until thawed.
5. Chocolate, despite being melted and heated during the production process, can freeze after being heated up. Once chocolate is melted and then rapidly cooled, it solidifies into a rigid structure, allowing it to maintain its shape when removed from the heat source and cooled even further.
1. Surprising Freezing Phenomenon
Introduction:
When we heat up a substance, it usually melts and turns into a liquid. However, there are certain substances that behave differently and actually freeze after being heated. This phenomenon has puzzled scientists and sparked interest in the world of phase changes. In this article, we will delve into the science behind this intriguing freezing phenomenon and explore the fascinating world of phase transitions.
The Freezing Phenomenon:
Normally, when heat is applied to a substance, its molecules gain energy and move more rapidly. This increase in energy causes the substance to transition from a solid to a liquid state, known as melting. However, certain substances undergo a peculiar process where they freeze when heated.
Understanding Phase Changes:
To comprehend this phenomenon, let’s first understand the concept of phase changes. Phase changes occur when a substance transitions between different states of matter – solid, liquid, and gas. The transition from solid to liquid is called melting, while the transition from liquid to solid is freezing.
Exceptional Substances:
While most substances melt when heated, there are a few exceptions that freeze instead. One example is a supercooled liquid. Supercooling occurs when a substance is cooled below its normal freezing point, but it remains in a liquid state due to the absence of nucleation sites. When heat is then applied to this supercooled liquid, it rapidly crystallizes, essentially freezing.
Key Factors:
Several factors contribute to the unique behavior of freezing after heating. One of these factors is the presence of impurities or foreign particles in the substance, which can act as nucleation sites and promote freezing instead of melting. Additionally, the rate at which heat is applied plays a significant role in determining whether the substance will freeze or melt.
Conclusion:
In conclusion, the freezing phenomenon after heating can be observed in certain substances, defying the conventional expectation of melting. Supercooled liquids are one example of substances that freeze when heated. Understanding the science behind this phenomenon requires exploring the concept of phase changes and considering factors like impurities and heat application rate. Discovering and studying these unique behaviors adds depth to our understanding of phase transitions.
Bullet Points:
- Substance melting when heated is a common phenomenon
- Some substances freeze instead of melting when heated
- The process of phase changes involves transitions between solid, liquid, and gas states
- Supercooled liquids are one example of substances that freeze when heated
- Impurities and heat application rate contribute to the freezing phenomenon
2. Heat-Induced Freezing
While it may seem counterintuitive, there are certain substances that freeze when exposed to heat. This phenomenon occurs due to the delicate balance between temperature and pressure. When a substance is subjected to increasing temperatures, its molecular structure can undergo significant changes. As a result, the substance may transition from a liquid to a gas, following a process called evaporation. However, under specific conditions, this process can be reversed, leading to the freezing of the substance.
3. Unexpected Frozen State
The frozen state that occurs after heating a substance defies our conventional understanding of thermodynamics. Thermodynamics is the branch of science that deals with the relationships between heat, energy, and work. According to the laws of thermodynamics, a substance should melt when heated and freeze when cooled. However, some substances exhibit an unusual behavior, contradicting these principles.
Despite our initial confusion, scientists have been able to explain this unexpected frozen state by studying the underlying molecular structure of the substances. By understanding the unique arrangements and interactions between atoms and molecules, we gain insights into the counterintuitive behavior of these substances.
4. Odd Thermodynamic Behavior
The odd thermodynamic behavior exhibited by substances that freeze after being heated up can be attributed to a phenomenon known as supercooling. Supercooling occurs when a substance is cooled below its freezing point without actually solidifying. This state is achieved under carefully controlled conditions, such as the absence of impurities or rapid cooling.
When supercooled substances are heated, they can momentarily exist in a “metastable” state, defying the typical rules of thermodynamics. However, due to the inherent instability of this state, the substance eventually reverts to its solid form, giving the illusion of freezing after being heated.
5. Curious Freezing Point
One intriguing aspect of substances that freeze after being heated up is their unique freezing point. The freezing point is the temperature at which a substance transitions from a liquid to a solid state. In most cases, substances freeze at lower temperatures as they cool down. However, substances with the peculiar behavior we are exploring in this article exhibit a higher freezing point after being heated.
This unusual characteristic challenges our understanding of how temperature affects the phase of matter. It highlights the complex interplay between molecular interactions and thermal energy, leading to unexpected freezing behavior.
6. Heat-Related Solidification
The process of solidification, or freezing, typically occurs when a substance loses thermal energy and transitions from a liquid to a solid state. However, the substances we are focusing on here defy this common understanding by solidifying after receiving additional thermal energy.
This unique heat-related solidification is the result of intricate molecular interactions that are still being studied by scientists. It involves a delicate balance between forces that drive a substance towards its solid state and those that promote a transition to a liquid or gaseous state. Understanding these mechanisms is a fascinating journey into the depths of thermodynamics and molecular physics.
- The process of solidification occurs when a substance loses thermal energy and transitions from a liquid to a solid state.
- However, certain substances solidify after receiving additional thermal energy.
- This unique heat-related solidification is the result of intricate molecular interactions.
- It involves a delicate balance between forces that drive a substance towards its solid state and those that promote transitions.
- Understanding these mechanisms is a fascinating journey into the depths of thermodynamics and molecular physics.
“Understanding these mechanisms is a fascinating journey into the depths of thermodynamics and molecular physics.”
7. Unusual Melting And Freezing
The melting and freezing behavior of substances that freeze after being heated up is far from ordinary. While most substances melt when heated and freeze when cooled, these exceptional substances display an unexpected reversal of this process.
When initially solid, these substances can be heated to transition into a liquid state. However, as the temperature continues to rise, they reach a point where they solidify again. This intriguing behavior challenges our preconceived notions of phase transitions and opens up new avenues of research in material science and thermal dynamics.
- These substances defy the traditional melting and freezing patterns observed in most materials.
- They transition from solid to liquid as they are heated, but then reverse this process and solidify again at higher temperatures.
- This behavior challenges our understanding of phase transitions and has sparked interest in material science and thermal dynamics research.
“This intriguing behavior challenges our preconceived notions of phase transitions and opens up new avenues of research in material science and thermal dynamics.”
8. Fascinating Phase Transition
The phase transition from a liquid to a solid state or vice versa is a mesmerizing process that occurs all around us. Whether it’s ice melting into water or wax solidifying upon cooling, phase transitions are fundamental to our daily lives. The substances that freeze after being heated up add another layer of complexity and fascination to this natural phenomenon.
Exploring the intricate details of phase transitions in these unique substances not only expands our knowledge of thermodynamics but also holds promising applications in various fields. From material science to engineering, understanding and harnessing these unconventional phase transitions may lead to innovative technologies and advancements.
9. Intriguing Frozen Characteristics
The frozen state that these substances adopt after being heated up carries distinctive characteristics. The resulting solid is often different from its original form, exhibiting modified physical properties and even altered molecular structures. This variation is a result of the complex interplay between heat and molecular interactions, further highlighting the mysterious nature of these substances.
By delving into the intricacies of this intriguing frozen state, scientists aim to unravel the underlying mechanisms and identify ways to manipulate and control it. This knowledge has the potential to revolutionize various industries, ranging from cryogenics to pharmaceuticals, where precise temperature control and solidification processes are crucial.
- The frozen state of substances after being heated up carries distinctive characteristics.
- Resulting solids often exhibit modified physical properties and altered molecular structures.
- The complexity of heat and molecular interactions contributes to the mysterious nature of these substances.
- Scientists are exploring this intriguing frozen state to understand its underlying mechanisms.
- Manipulating and controlling the frozen state could have significant implications for industries such as cryogenics and pharmaceuticals.
- Precise temperature control and solidification processes are crucial in these industries.
10. Puzzling Thermal Change
The thermal change observed in substances that freeze after being heated up remains a captivating puzzle for scientists to solve. While significant progress has been made in understanding the underlying mechanisms, there is still much to uncover. From studying the molecular interactions to analyzing the role of impurities and external factors, researchers strive to piece together the intricate puzzle of this enigmatic thermal behavior.
As our understanding of these substances deepens, we can glimpse the magnificent complexity of the natural world. The quest to unravel the secrets of what freezes after being heated up continues to captivate the scientific community, driving advancements and pushing the boundaries of our knowledge.
The phenomenon of substances freezing after being heated up challenges our conventional understanding of phase transitions and thermodynamics. By studying the intricate molecular interactions and delicate balance of forces, scientists are unraveling the mysteries behind this unique behavior. This exploration of what freezes after being heated up opens up new avenues of research and holds potential applications in various fields. The journey to comprehend the complex thermal changes exhibited by these substances is a testament to the never-ending quest for knowledge and understanding in the scientific community.
💡
You may need to know these questions about what freezes after it is heated up
Which thing freezes after heating?
GELATIN: Surprisingly, gelatin is another thing that freezes after heating. When heated, gelatin turns into a liquid form, but as it cools down, it solidifies and freezes, creating a gel-like consistency. This property is what makes gelatin the perfect ingredient for creating delicious desserts such as jelly or panna cotta.
1. What substance freezes after it is heated up and can be commonly found in kitchens?
One substance that freezes after being heated up and is commonly found in kitchens is water. Water is known to freeze when it reaches its freezing point of 0 degrees Celsius (32 degrees Fahrenheit). This can happen if water is heated and then rapidly cooled, such as when it is placed in a freezer, resulting in the formation of ice. Ice can be found in various forms in the kitchen, whether it is used for cooling drinks or as an ingredient for recipes that require frozen substances.
Another substance that can freeze after being heated up and is common in kitchens is fat or oil. When heated, fats and oils tend to melt and become liquid. However, if they are then cooled down, they solidify and form a frozen or semi-solid state. This can be observed when we cook food with oils or keep sauces containing fat in the refrigerator, resulting in solidification and giving the food a frozen or thicker texture.
2. Can you explain the scientific phenomenon behind why certain liquids freeze when heated?
When certain liquids freeze when heated, it is usually due to a phenomenon called supercooling. Supercooling occurs when a liquid is cooled below its freezing point without actually solidifying. This happens because ice crystals need a nucleation point to form, typically provided by impurities or a rough surface. However, in a supercooled liquid, these nucleation points are absent, so the liquid remains in a seemingly liquid state even below its freezing point.
When this supercooled liquid is then heated, it can undergo rapid crystallization or solidification. The heating causes the molecules in the liquid to move more vigorously, breaking the supercooling state. The introduction of energy disrupts the balance of forces between the liquid molecules, prompting them to reorganize into a solid structure, forming ice crystals. This sudden phase transition from liquid to solid results in the liquid appearing to freeze when heated.
3. Are there any practical uses or applications for substances that freeze after being heated?
Yes, there are practical uses and applications for substances that freeze after being heated. One example is shape memory alloys (SMAs), which are special materials that can change shape when heated and then revert back to their original shape when cooled. This property makes them useful in various fields such as medicine, aerospace, and robotics. In medicine, SMAs can be used in orthodontics to create self-adjusting braces that gradually straighten teeth. In aerospace, these alloys have applications in constructing materials that can withstand extreme temperatures, while in robotics, they can be used in developing flexible and adaptable devices.
Another practical application is found in self-healing materials. These substances have the ability to repair damage caused by external factors, such as heat or mechanical stress. When heated, the material melts and flows into the damaged area, filling cracks and gaps. Once cooled, it solidifies and binds, effectively sealing the damage. Self-healing materials have potential applications in various industries, including construction, automotive, and electronics. For example, in the construction industry, self-healing concrete could help increase the durability and lifespan of structures by repairing cracks caused by temperature fluctuations or seismic activity.
Reference source
https://www.puzzleprime.com/puzzles/casual-puzzles/riddle/freezes-when-overheated/#:~:text=The%20answer%20is%20COMPUTER.
https://www.quora.com/Which-substance-is-freezed-on-heating#:~:text=Originally%20Answered%3A%20What%20is%20such,lower%20than%20that%20of%20water.
https://riddles.net/what-freezes-after-it-is-heated-up
https://logiclovely.com/p/what-freezes-after-it-is-heated-up/