Heated Liquid: The Surprising Science Behind Boiling
- The article discusses the importance of specific heat when heating liquids.
- Specific heat is the amount of energy required to increase the temperature of a substance by 1 degree.
- The specific heat of water is 1.0 BTU/lb °F, while the specific heat of salt is 0.21 BTU/lb °F.
- Knowing the specific heat is crucial in calculating the required energy for heating a liquid.
- For example, heating 1 cubic foot of water from 68°F to 120°F in 3 hours requires 500 watts of energy, whereas heating the same volume of #2 oil with a specific heat of 0.44 BTU/lb °F only requires 351 watts.
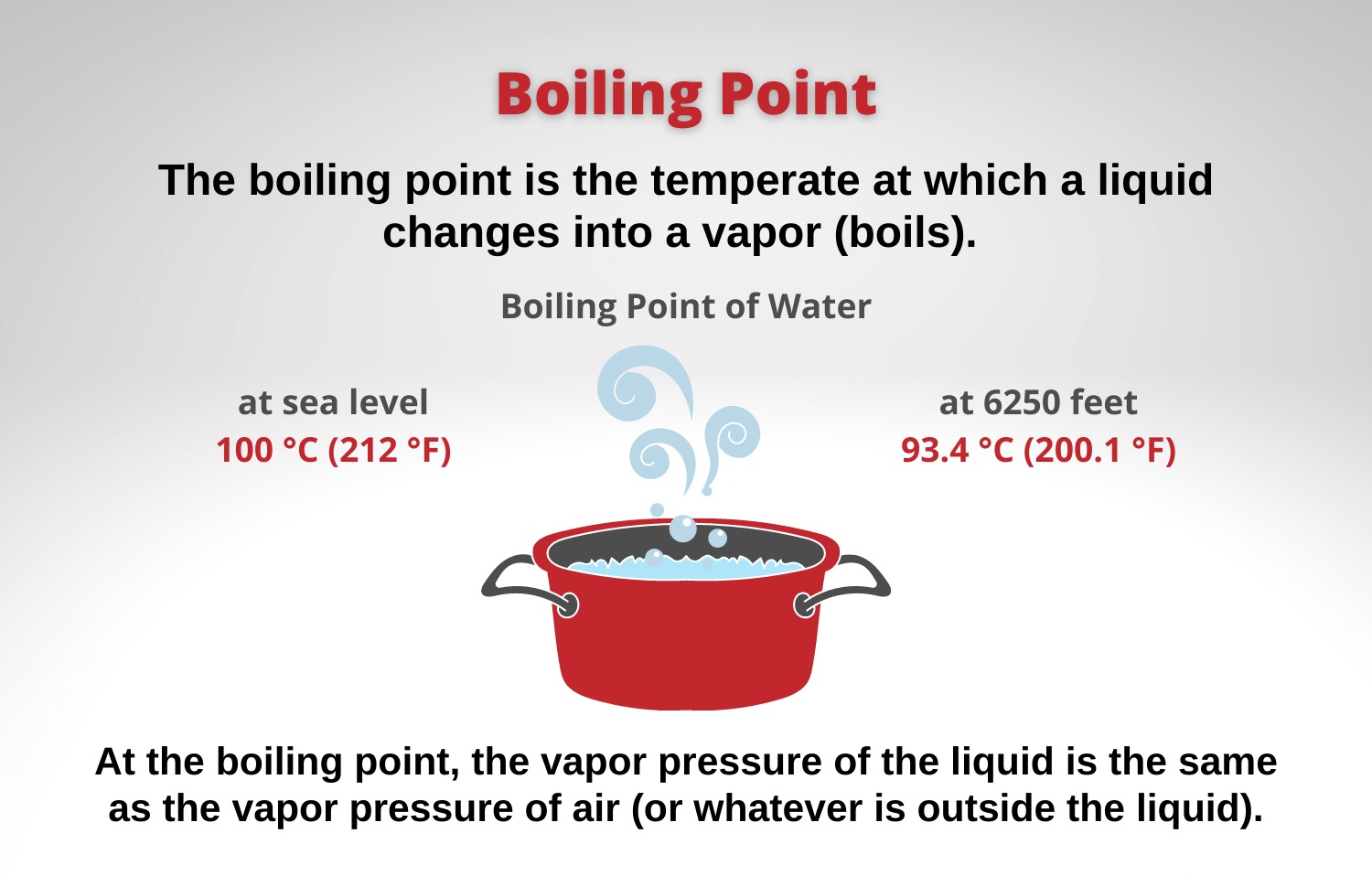
- Adding salt to water lowers its specific heat, resulting in a faster boiling time.
- Conversely, if a system designed to heat a fluid with a lower specific heat is used to heat a fluid with a higher specific heat, it may not reach the desired temperature or require more time.
- The article also mentions BriskHeat's heat loss calculator as a tool to help with calculating heat loss in fluid heating processes.