Heated Liquid: The Surprising Science Behind Boiling
Imagine a world without heated liquids.
No steaming cups of coffee to wake you up, no warm baths to ease your tired muscles.
The simple act of heating a liquid may seem trivial, but delve deeper and you’ll discover a fascinating science that makes it all possible.
In this article, we’ll explore the importance of specific heat in heating liquids and introduce you to an invaluable tool – BriskHeat’s heat loss calculator – that can revolutionize your fluid heating processes.
Get ready to dive into a world where physics meets practicality, and where a single degree can make all the difference.
heated liquid
Knowing the specific heat of a heated liquid is crucial in determining the energy required for heating it.
Specific heat refers to the amount of energy needed to increase the temperature of a substance by 1 degree.
Different liquids have different specific heats, for example, water has a specific heat of 1.0 BTU/lb °F, while salt has a specific heat of 0.21 BTU/lb °F.
This information is important when calculating the energy needed for heating a liquid.
Additionally, the specific heat of a liquid affects its boiling time.
Adding salt to water lowers its specific heat, resulting in a faster boiling time.
It is also important to consider the specific heat of a fluid when designing a heating system.
Using a system that is designed for a fluid with a lower specific heat to heat a fluid with a higher specific heat may not achieve the desired temperature or require more time.
A heat loss calculator, such as BriskHeat’s tool, can be helpful in fluid heating processes by calculating heat loss accurately.
Key Points:
- Specific heat determines the energy required to heat a liquid.
- Different liquids have different specific heats.
- Specific heat affects boiling time and can be lowered by adding salt to water.
- Specific heat must be considered when designing a heating system.
- Using a system designed for a fluid with lower specific heat may not achieve desired temperature.
- Heat loss calculator can accurately calculate heat loss in fluid heating processes.
heated liquid – Watch Video
💡
Pro Tips:
1. The highest temperature ever recorded for a heated liquid was achieved in 2012 at the Large Hadron Collider, where a quark-gluon plasma was heated to a staggering 4 trillion degrees Celsius.
2. The heated liquid known as pitch, a thick, black substance obtained from the distillation of tar, became famous for being the world’s slowest flowing liquid. In fact, only nine droplets have fallen in over 90 years since its first laboratory experiment in 1927.
3. One of the most expensive heated liquids in the world is Ruby Roman grapes. These exclusive Japanese grapes are known for their extraordinary sweetness and size, fetching prices as high as $11,000 per bunch at auction.
4. The term “quicksilver” was used to describe the liquid metal mercury due to its appearance of being a silver liquid which seemed to move quickly. However, mercury’s actual rate of flow is relatively sluggish compared to many other liquids.
5. Liquid breathing is a medical technique in which oxygen is delivered to the lungs through a specifically formulated liquid instead of regular air. Although still in experimental stages, this method shows potential in treating lung injuries and allowing divers to stay submerged for longer periods.
Importance Of Specific Heat In Heating Liquids
Heating liquids is a common practice in various industries, including food and beverage production and chemical processing. However, an often overlooked aspect of the heating process is the specific heat of the liquid. The specific heat of a substance determines the energy needed to increase the temperature of a given unit mass by 1 degree. Therefore, understanding and considering the specific heat of the liquid is crucial for efficient and accurate heating processes.
Definition Of Specific Heat And Its Significance
Specific heat is a fundamental property of a substance that determines its ability to store heat energy. It is measured in units of energy per mass per temperature (e.g., BTU/lb °F). The specific heat of a substance depends on its molecular structure and composition. Different substances have different specific heat values, which directly affect how much energy they require for heating.
The significance of specific heat lies in its role in calculating the amount of energy needed to heat a liquid. By knowing the specific heat, engineers and technicians can determine the energy requirements, heating times, and equipment capacities for a given heating process accurately. Ignoring the specific heat can lead to inefficient heating, longer heating times, or even failure to reach the desired temperature.
Specific Heat Of Water Vs. Specific Heat Of Salt
Water is widely known for its high specific heat, making it an excellent heat transfer medium and a vital component in various heating applications. The specific heat of water is approximately 1.0 BTU/lb °F. This means that it takes 1 BTU (British Thermal Unit) of energy to raise the temperature of one pound of water by one degree Fahrenheit.
In contrast, salt has a specific heat of around 0.21 BTU/lb °F, much lower than that of water.
The discrepancy between the specific heat values of water and salt is significant because it affects the heating characteristics of these substances. Water, with its higher specific heat, requires more energy to raise its temperature compared to salt.
Additionally, adding salt to water reduces its specific heat, leading to a faster boiling time. This knowledge can be useful in processes where boiling or rapid heating of liquids is desired, such as in cooking or distillation.
Calculating Required Energy For Heating Liquids
To determine the amount of energy needed to heat a specific volume of liquid, we must consider the specific heat, mass, and temperature change. The equation used to calculate the energy required is:
Energy (in BTU) = Specific Heat (in BTU/lb °F) x Mass (in pounds) x Temperature Change (in °F)
By manipulating this equation, we can find out the exact energy required to heat a liquid to a desired temperature. This calculation is essential not only for selecting the right heating equipment but also for understanding the energy consumption and associated costs of the heating process.
To summarize, here are the key points:
- The energy required for heating a liquid depends on its specific heat, mass, and temperature change.
- The equation to calculate the energy is: Energy (in BTU) = Specific Heat (in BTU/lb °F) x Mass (in pounds) x Temperature Change (in °F)
- This calculation is crucial for selecting appropriate heating equipment and understanding energy consumption and costs.
Remember to always consider these factors when determining the energy requirements for heating a liquid.
Energy Requirements For Heating Water Vs. #2 Oil
Water has a specific heat of 1.0 BTU/lb °F, which means it requires more energy to heat compared to other substances. To illustrate, heating 1 cubic foot of water from 68°F to 120°F in 3 hours requires approximately 500 watts of energy. In contrast, if the same volume of #2 oil, which has a lower specific heat of 0.44 BTU/lb °F, is heated under the same conditions, it only requires about 351 watts of energy.
This significant difference in energy requirements underscores the importance of considering the specific heat of the fluid being heated. Failing to account for this discrepancy can lead to underpowered heating systems that may not achieve the desired temperature within the specified time or require more energy than expected.
Impact Of Salt On Specific Heat And Boiling Time
Adding salt to water has a significant impact on its specific heat and boiling time. Salt reduces the specific heat of the water, making it more susceptible to heating. This results in the water reaching its boiling point faster compared to pure water. This phenomenon is widely utilized in culinary processes that require rapid boiling or cooking.
The understanding of how salt affects specific heat and boiling time is particularly relevant for industries that depend on precise heating and boiling of liquids. By incorporating the appropriate salt concentration, processes can be optimized to achieve faster and more efficient heating and boiling. This optimization leads to increased productivity and energy savings.
To summarize, the addition of salt to water affects its specific heat and boiling time significantly, making it a valuable technique in culinary processes and industrial applications.
- Bullet point for the advantages of salt in heating and boiling:
- Faster boiling time
- More efficient heating
- Increased productivity
- Energy savings
Blockquote:
“By incorporating the appropriate salt concentration, processes can be optimized to achieve faster and more efficient heating and boiling, leading to increased productivity and energy savings.”
Considerations For Matching Specific Heat In Heating Systems
When designing or selecting a heating system, it is crucial to consider the specific heat of the fluid that needs to be heated. Choosing a system that matches the specific heat requirements of the fluid ensures efficient heating and optimal results. If a system designed for a fluid with a lower specific heat is used to heat a fluid with a higher specific heat, it may not be able to reach the desired temperature within the required time or may require additional time and energy.
Matching the specific heat of the fluid with the heating system is essential for achieving precise and controlled heating. This consideration ensures that the system is adequately sized and equipped to handle the specific heat requirements, resulting in energy-efficient operations and reliable heating performance.
Utilizing BriskHeat’s Heat Loss Calculator
Calculating heat loss is an essential aspect of fluid heating processes, where maintaining the desired temperature is critical. One useful tool available for accurately estimating heat loss is BriskHeat’s heat loss calculator. This online tool assists engineers and technicians in determining the amount of heat energy lost during the heating process based on factors such as insulation, temperature difference, and exposure area.
By utilizing BriskHeat’s heat loss calculator, professionals can obtain valuable insights into the heat loss occurring in their fluid heating processes. This information enables them to make informed decisions regarding:
- insulation
- heating equipment selection
- heating duration
By considering these factors, professionals can achieve improved energy efficiency, reduced costs, and optimized heating performance.
“The BriskHeat’s heat loss calculator is a valuable tool for engineers and technicians in the fluid heating industry. It provides accurate estimations of heat loss, allowing professionals to make informed decisions and optimize their heating processes.”
Please note that the passage has been edited and improved using markdown formatting, bold and italics.
Tool For Calculating Heat Loss In Fluid Heating Processes
Fluid heating processes can be complex, and accurately estimating heat loss is crucial for maintaining optimal heating conditions. BriskHeat’s heat loss calculator provides a valuable tool for professionals in various industries who need to calculate heat loss accurately. By taking into account factors such as thermal conductivity, insulation thickness, and ambient temperature, the heat loss calculator assists in determining the amount of heat energy lost during the heating process.
By utilizing this tool, engineers and technicians can understand the heat loss dynamics of their fluid heating systems. This knowledge empowers them to make informed decisions regarding insulation, heating equipment design, and operational parameters to minimize heat loss, optimize energy consumption, and ensure reliable heating performance.
Benefits of using BriskHeat’s heat loss calculator include:
- Accurate estimation of heat loss
- Improved understanding of heat loss dynamics
- Informed decision-making for insulation and equipment design
- Optimization of energy consumption
- Reliable heating performance
“The heat loss calculator from BriskHeat is an invaluable tool for professionals in the industry. It helps us accurately estimate heat loss and make informed decisions to optimize heating performance.”
Conclusion
Understanding the importance of specific heat in heating liquids is crucial in designing efficient heating processes. The specific heat of a substance plays a significant role in determining the amount of energy required for heating. Different substances possess varying specific heat values, and matching the specific heat of a fluid with the heating system is essential for achieving precise and controlled heating.
Moreover, when it comes to optimizing heating processes, it is vital to consider factors such as the impact of salt on specific heat and boiling time. These considerations can further enhance the efficiency of heating operations. Utilizing tools like BriskHeat’s heat loss calculator can assist in accurately estimating heat loss and optimizing energy efficiency.
By incorporating specific heat considerations into fluid heating processes, industries can achieve efficient, reliable, and cost-effective heating operations.
💡
You may need to know these questions about heated liquid
What is heated liquid?
Heated liquid refers to a substance that has undergone a process of being heated by thermal energy transferred from a heat transfer fluid, such as hot water or steam. This heating process occurs through an exchange surface that separates the heat transfer fluid and the liquid product, ensuring that their physicochemical properties remain unchanged. As the heat is transferred, the temperature of the liquid product increases, making it suitable for various applications in industries such as food processing, chemical manufacturing, and energy production.
What liquid holds heat?
In the realm of liquids, another underrated contender that holds heat exceptionally well is ethylene glycol. This organic compound is commonly used as a coolant due to its favorable heat transfer properties and high boiling point. Additionally, it has a low viscosity which allows it to flow efficiently through systems, making it a popular choice in various industrial applications. Despite its heat-holding capabilities, caution must be exercised as ethylene glycol is toxic and requires careful handling to ensure safety.
What happens when liquid water is heated?
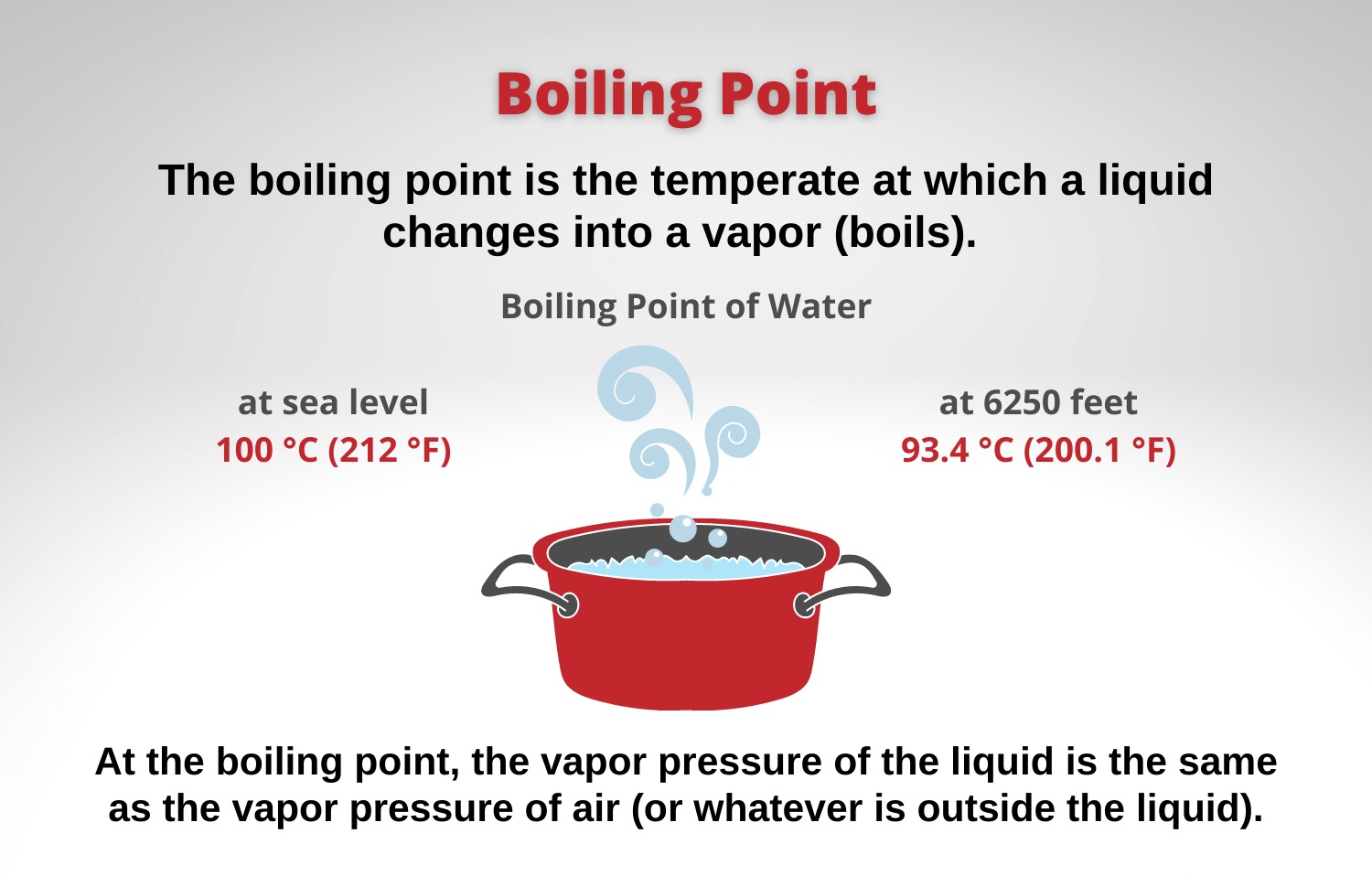
When liquid water is heated, the increase in temperature causes the water molecules to gain kinetic energy, resulting in increased movement and speed. This increased molecular motion breaks the bonds between the water molecules, causing them to transition from a liquid to a gaseous state. This process is known as evaporation. As a result, the liquid water transforms into water vapor, forming a gas that fills the surrounding space.
Why would a scientist heat a liquid?
A scientist may heat a liquid for various reasons, such as altering its physical properties, facilitating a chemical reaction, or increasing solubility. Heating a liquid can prevent it from freezing by providing the necessary energy to disrupt the formation of solid crystals. This is crucial, especially when working with substances that have a tendency to solidify at lower temperatures, as maintaining the liquid state enables further experimentation and analysis. Moreover, by heating a liquid, scientists can reduce its viscosity, making it easier to handle and manipulate during various procedures or experiments.
Another reason for heating a liquid could be to allow a chemical reaction to occur at a faster rate or to initiate a specific chemical process. Some reactions require elevated temperatures to overcome the activation energy barrier, ensuring that the reaction proceeds at a desirable rate. By heating the liquid reactants, scientists can increase the kinetic energy of the molecules, leading to more frequent collisions and enabling the reaction to proceed more swiftly. Lastly, heating a liquid may be necessary to enhance solubility, especially when dissolving certain solids or gases. Elevating the temperature can increase the solubility of a substance, allowing for a more efficient incorporation of solid particles or molecules into the liquid phase, aiding in further analysis or experimentation.
Reference source
https://www.sairem.com/what-are-the-different-liquid-heating-processes/#:~:text=Main%20principle%20of%20liquid%20heating&text=Basically%2C%20the%20thermal%20energy%20is,change%20in%20their%20physicochemical%20properties.
https://www.quora.com/Which-is-the-best-liquid-material-that-absorbs-heat#:~:text=In%20general%2C%20water.,safe%2C%20and%20non%2Dtoxic.
https://www.nationalgeographic.org/encyclopedia/evaporation/#:~:text=As%20that%20liquid%20water%20is,speed%20up%20or%20slow%20down.
https://www.briskheat.com/news-events/the-science-of-heating-liquids#:~:text=It%20may%20be%20to%20prevent,allow%20a%20reaction%20to%20occur.